Packing with VCIs and how to apply them
VCI – Volatile Corrosion Inhibitor is formed of volatile organic compounds that inhibit the corrosion process on metallic materials, not causing harm to the environment. This process happens with the volatization of these low vapor pressure compounds and the deposition of a protective layer on the metallic surface.
This barrier prevents any oxidation to take place on the metal surface. This vapor doesn’t deposit a film or residue on the metal surface, eliminating the need for expensive, long, unhealthy and polluting methods of applying and removing conventional oily and greasy protectors.
See below the methods of application:
PAPER
VCI Brasil Paper is a special neutral kraft
paper with high absorbency that is chemically treated in one side.
PLASTIC FILMS
The VCI691 and FM8 films (Stretch Film) from VCI Brasil made of Polyethylene resins or combination with other resins.
They are produced by mono-extrusion (one layer) or co-extrusion (three layers) in cast or blow machines.
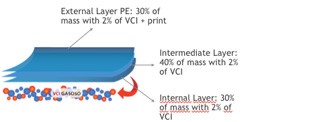
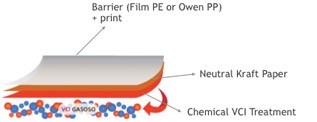
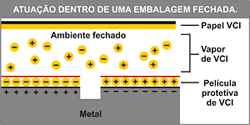
Action on the packaging
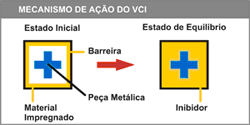
The organic base acts as a passive and active carrier, that, when volatized, carries an organic or inorganic anion (negatively charged molecule). This anion tends to naturally deposit on the metal surface forming a uniform and invisible film.
The VCI continues to volatize and when a certain concentration of vapor is reached, equilibrium is established, resulting in a perfect trade-off between the inhibitor on the package and the inhibitor on the metal surface – the vapor at the same speed as the VCI volatizes, as shown in the figure above.
Ideal Package
In an ideal system for the occurrence of protection, the package must be airtight bringing the environment to a state of equilibrium. In this case the durability of anticorrosive protection with VCI tends to be infinite. If there is an exchange with the outside (small leaks or vents) there will be a constant 'renewal' of the environment, which causes consumption and consequently wear of the VCI implemented in the system.
The inhibitory layer of protective ions on the metal surface is preserved while the product remains enclosed in the package. The protection mechanism ensures that the parts are ready for immediate use without the need of cleaning or washing with solvent.
This additional cleaning operation depends mainly on the final application of the metal in question. The metallic corrosion begins when moisture (that contains an electrolyte) condenses on the metal surface and causes a flow of electrons between the metal surface and the electrolyte. The VCI molecules do not prevent the deposition of the electrolyte, however directs the existing flow in the desired orientation so that there is no oxidation of the metal. Depending on the metal and the inhibitor system, the thin layer formed physically prevents the contact of the electrolyte with metal.
Temporary Anticorrosive Protection
Some metallic products need temporary protection because their surfaces need to show no alteration after some storage and/or transportation time.
Temporary in this case means that the eventual protection must be easily removed leaving the metallic surfaces unaltered for the next step. Not the time the protection lasts.
The main causes of corrosion are:
- Contamination by corrosive products (during production and handling).
- Packages containing corrosive substances.
- Atmospheric Corrosion.
Further than appropriate packages, at times the package interior must prepared with a temporary protective product.
Temporary protection is generally obtained via acclimatization of the environment, the used of products that form a protective layer, use of special packaging containing corrosion inhibitor or by combination of these methods.
The flowchart bellow illustrates these methods.
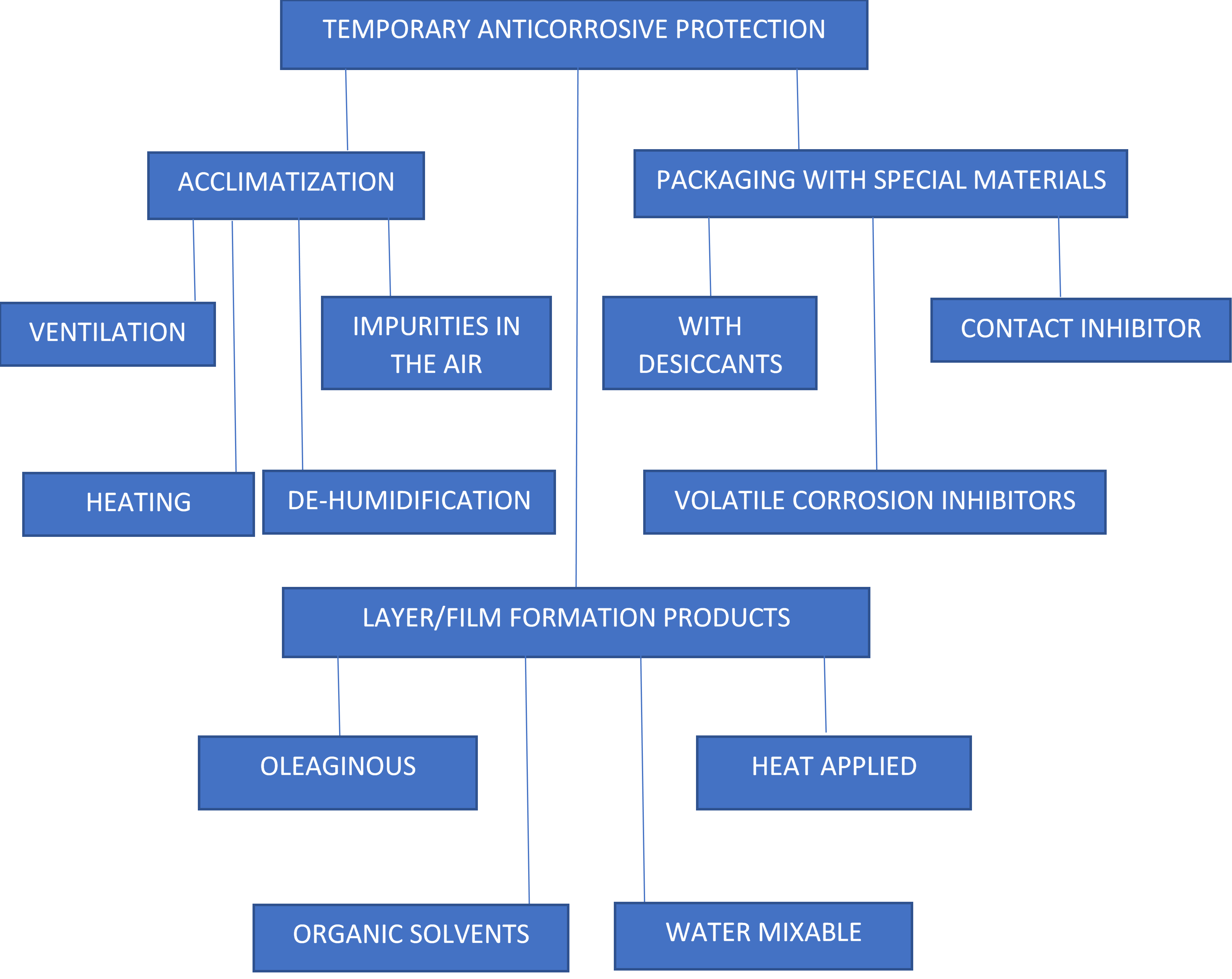
ACCLIMATIZATION:
Deposition of a layer of water (condensation) on the metallic surface is the main contributing factor to the corrosion process.
The main methods to prevent water condensation are:
Ventilation: Used in environments where humidity and temperature controls are difficult to implement.
This is done by natural (air drafts) or forced ventilation (use or fans) and in combination, hanging the subjects at least 1m from the walls.
Heating: This is done only where there is a large temperature variation between day and night.
Heat is used to control the relative humidity keeping it always close to 60%.
For indoors, heaters ale placed high on the walls and exhaust fans on the lower parts of the walls so there is real air renovation.
It is important to be careful about the choice of heaters because some combustion heaters emit gases such CO2 and SO2, that are highly corrosive.
Humidity must be controlled by a hygrometer.
De-humidification: Only used when the other two methods alone don’t solve the humidity problem.
The most common processes of de-humidification are refrigeration or desiccants (Silica gel or Lithium Chloride).
Elimination of impurities in the air:
Used in environment with excess of combustion emission (CO2, SO2, soot etc.) acidic vapors and others.
Protective Film Formation:
Use of products that form a superficial film that prevents the contact on the metallic surface and corrosive substances.
The layer or film is thick, depending on the level of protection.
Usually these are petroleum derivates, oils, greases that with substances that promote a uniform layer in addition to the corrosion inhibiting chemicals
The disadvantage of this process is in removing process that is difficult, costly, unhealthy and inefficient. To remove these protective layers organic solvents, such as aromatic and/or chlorinated are used. These solvents are highly toxic to humans and the environment.
These protective products are:
LAYER/FILM FORMATION PRODUCTS: oils, greases, resins, Vaseline etc.;
Solvents: Water, organic substances etc.
Corrosion Inhibitor: Polar compounds of sulfur and nitrogen.
Acidic neutralizers.
These were classified in:
Water Mixable – May be soluble or produce emulsions with water.
Oleaginous
Organic Solvents – oily, greasy or waxy, varnish and plastic
Heat applied – Vaseline, wax thermoplastics.
PACKAGING WITH SPECIAL MATERIALS
This method is used the most due to its versatility and ease of application. It is an excellent mechanical and anticorrosive agent for storage or transportation.
These types of packages differ mostly by the protective agent used:
With Desiccants: chemical compounds that eliminate the humidity inside the package, such as Silica Gel or Lithium Chloride
Used when other protective substances because of chemical incompatibility or as a reinforcement as in electronic equipment.
Contact Inhibitors: these products act only in direct contact with the metallic surface; contain inorganic salts impregnated to paper normally with Sodium Nitrite.
Volatile Corrosion Inhibitors: these substances when utilized, volatize and reaching the metallic surface form an invisible film.